The New Neutralization Treatment Facility
The New Neutralization Treatment Facility (former Matsuo Mine)
Treatment with Iron Oxidizing Bacteria
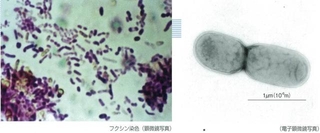
The new neutralization treatment facility is characterized through its use of bacteria to oxidize the iron in the wastewater so it can neutralize the water with calcium carbonate.
Usually, slaked lime is used to neutralize acidic wastewater with a high quantity of bivalent Fe2+ ions. However, when treating large volumes of wastewater (18m3/min) like at the Matsuo Mine, the use of slaked lime (a relatively expensive material) would drive treatment costs too high.
The wastewater at Matsuo Mine is acidic, with a high concentration of bivalent Fe2+ ions, and a large amount of water must continually be treated and neutralized. Because of this, the method of using bacteria to oxidize and calcium carbonate to neutralize to wastewater is the appropriate method for the neutralization facility.
Calcium carbonate is cheaper than slaked lime and reacts easily to trivalent Fe3+ ions, but is not suitable for Fe2+ ion reactions – which, unfortunately, the Matsuo Mine wastewater is full of. However, iron oxidizing bacteria have the ability to oxidize Fe2+ ions into Fe3+ ions, so the bacteria is used to convert the wastewater into wastewater high in Fe3+ ions. This wastewater can then be treated using the cheap neutralizing agent calcium carbonate.
When Fe2+ ions oxidize, the energy produced is used by the iron oxidizing bacteria (250,000 bacteria per 1ml of wastewater). The neutralization facility injects air into the wastewater to stimulate the bacteria, which then multiply into 100 million bacteria per 1ml of wastewater.This high concentration is maintained to continually oxidize the ions in the wastewater.
The permanent drainage tunnel

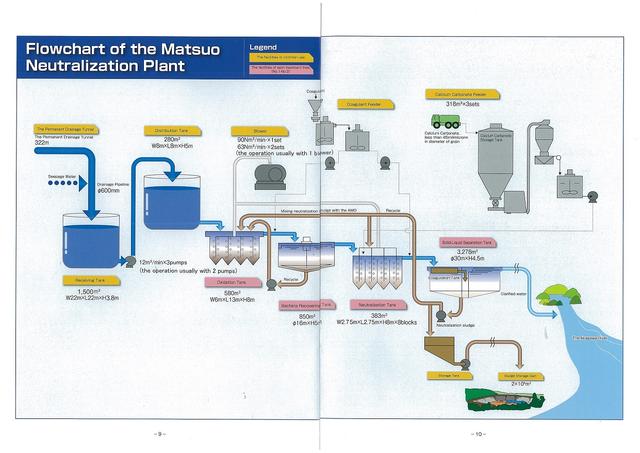
The permanent drainage tunnel collects wastewater from the mine shaft and routes it to the neutralization treatment area.Construction of the tunnel was completed in March 1984, taking around one and half years at a cost of \600,000,000.
The total length of the concrete tunnel is 322m and at its center is a rigid vinyl chloride pipe with a diameter of 600mm. The wastewater is fed along this pipe to the raw water receiving tank and from there sent to a raw water distribution tank.
Raw Water Distribution Tank

This tank equally distributes the wastewater from the permanent drainage tunnel to each treatment section.
At the facility, treatment areas 1-3 have a max treatment capacity of 12m3/min, and there is a 4th treatment area for emergencies (with a treatment capacity of 9m3/min). The wastewater is distributed in this way to be treated.
Oxidation Tank

This tank oxidizes the bivalent Fe2+ ions contained in the wastewater to trivalent Fe3+ ions.
Exposing the wastewater to air adds oxygen from the air to the water. Iron oxidizing bacteria use that oxygen to oxidize bivalent Fe2+ ions into trivalent Fe3+ ions and gain energy from the process.
The bacteria use that energy in order to multiply as well as maintain a high concentration in the carrier for the bacteria. The carrier consists of the sediment produced through neutralization reactions.
The reaction below occurs due to the actions of iron oxide bacteria maintained at high concentrations in the tank.
Reaction formula
4FeSO4(monohydrate of ferrous sulfate)+2H2SO4(sulfuric acid)+O2(oxygen)
→2Fe2(SO4)3(ferric sulfate)+2H2O(water)
Bacteria Collecting Tank

This tank precipitates out the sediment after it has clumped together and return it to the oxidation tank as the carrier for the bacteria.
The sediment is collected from the bottom of the collection tank and sent back to the oxidation tank, while the water floating on top is taken to the neutralization tank using a downward flow.
By the time the water is sent to the neutralization tank, almost all of the Fe2+ ions in the wastewater have been oxidized to Fe3+ ions.
Neutralization Tank

This tank neutralizes the oxidized wastewater with calcium carbonate.
The water is neutralized as follows: a milky calcium carbonate solution is added to the tank, and the solution is then agitated through air being pumped into the wastewater.
A large amount of iron-containing sediment is produced in this reaction.
Reaction formula
Fe2(SO4)3(ferric sulfate)+3CaCO3(calcium carbonate)+3H2O(water)
→2Fe(OH)3↓(ferric hydroxide)+3CaSO4↓(calcium sulfate)+3CO2↑(carbon dioxide)
Solid-Liquid Separating Tank

Water flows from the neutralization tank into this tank, where the solution is separated into sediment and supernatant water.
A polymer flocculant agent is added to the water in order to quickly sink the sediment produced from neutralization.
At this point, some sediment has already been precipitated out and sent to be used in the oxidation tank and neutralization tank, but from this point the remaining sediment produced from neutralization is sent to the waste dam and the supernatant water is discharged into the Aka River.
Waste Dam

This dam collects the sediment produced from neutralization.
The old Hikage-numa Pond adjoining the treatment facility was dredged and dug out to construct an inclined impervious core fill-type dam. The east-west embankment is made using the natural topography. The north-south embankment is made using earth and rocks taken from the west side of the site.
The inner side of the embankments are made using the rip-rap method of construction where rocks are attached to the surface of the embankment to prevent erosion.
The capacity of the dam is 2,000,000m3 and currently about 500,000m3 of sediment has been deposited here, equaling about 20,000m3 of sediment deposited per year (volume when wet).
PDFファイルをご覧いただくには、「Adobe(R) Reader(R)」が必要です。お持ちでない方はアドビシステムズ社のサイト(新しいウィンドウ)からダウンロード(無料)してください。
このページに関するお問い合わせ
Mining and Water Resources Section (Mining), Environmental Preservation Division,
Department of Environment and Residential Life
(020-8570) 10-1 Uchimaru, Morioka, Iwate, JAPAN
TEL:019-629-5358 FAX:019-629-5364

